
This week I spoke at two literary events in London. What could a medical translator bring to the literary translator’s table? Read on to find out.

This week I spoke at two literary events in London. What could a medical translator bring to the literary translator’s table? Read on to find out.


When I was a tutor on the Setting up as a freelance translator course run by the Institute of Translation and Interpreting (ITI), I tried to instil in my students the importance of preparing for the unexpected. Always have a backup plan in place – I advised them – you never know when you might need it. And guess what? My backup plan was put to the test last March.
Every other year, Trados developers announce a new Studio version in our beta-testing forum and we enthusiastically download a series of builds over the course of several weeks, until a fully fledged release candidate appears, ready for launch to the wider world. Trados Studio 2022 has arrived, two years since Trados Studio 2021 appeared on the scene. Here is a review of the changes it brings, starting with the disappearance of the name-date paradox.
Continue readingAs a medical translator with a background in nursing, I was delighted when my son Leo chose to train as an emergency medical technician (EMT) and join the Madrid ambulance service. He’s shown me round various ambulances and we often chat about trauma, technology and terminology. This March, I got the opportunity to find out how his experiences, and the terms he and his colleagues use, differ from their counterparts in the UK. I stepped on board a UK ambulance for a guided tour by my friend Jon Murrell, a paramedic practitioner stationed at Redhill, in Surrey. We spent the afternoon packing and unpacking equipment, locking and unlocking drug storage systems, opening and closing carry chairs and other transfer devices, and over a coffee we thrashed out the pros and cons of English and Spanish ambulance crew training, logistics and communications.

A new version of AntConc was released at the end of last year. I spotted the announcement on Twitter and was quick to download and start testing the tool, taking advantage of some downtime over the holidays. Read on for a report on my initial findings, some delightful discoveries and the odd little niggle.
 The Keyboard Corner is back! Today’s keyboard review is by Kit Cree, a French and Spanish to English translator. Kit is reporting on her favourite keyboard, a mechanical keyboard by KROM for gamers – and translators and editors, too! Read more…
The Keyboard Corner is back! Today’s keyboard review is by Kit Cree, a French and Spanish to English translator. Kit is reporting on her favourite keyboard, a mechanical keyboard by KROM for gamers – and translators and editors, too! Read more…
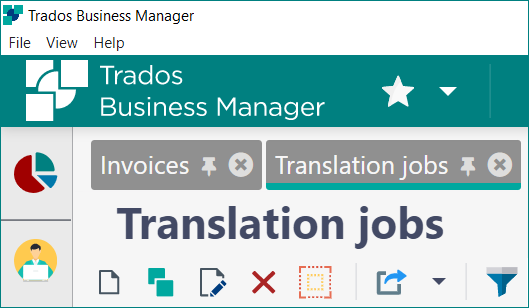
Although RWS acquired SDL last November, the visible switch from gaudy green to tranquil teal happened just a few weeks ago. And along with the rebranding, we have a reinvented app: Trados Business Manager Essential.
Last week a colleague asked the Twittersphere whether endometriosis is a disease, disorder or condition. Always keen to accept a challenge, I went off to investigate. Join me on my journey down the rabbit hole!