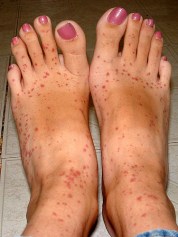
Adverse Drug Reaction reporting
EMA, the European Medicines Agency, has recently launched a new website giving public access to the European database of suspected adverse drug reaction (ADR) reports. The website provides an A-Z list of reports that have been submitted to EudraVigilance by national medicines regulatory authorities. The list is classified by product and substance.
For medical translators the new website offers a good opportunity to brush up on terms and definitions used in ADR reporting, so the rest of this post is a brief review of ADR terminology in the form of a Q&A section. Read more…