 A new version of the Medical Dictionary for Regulatory Activities (MedDRA) has just been released. This blog post will provide a brief introduction to MedDRA and next week I’ll look at some of the changes in version 19.0, published in English on 2 March and in other languages today.
A new version of the Medical Dictionary for Regulatory Activities (MedDRA) has just been released. This blog post will provide a brief introduction to MedDRA and next week I’ll look at some of the changes in version 19.0, published in English on 2 March and in other languages today.
What is MedDRA?
MedDRA is the acronym for Medical Dictionary for Regulatory Activities. It’s an international medical terminology used by the pharmaceutical industry, medical device industry and regulatory agencies throughout the drug development process, in clinical trials and for postmarketing activities such as pharmacovigilance (periodic safety update reports and adverse drug reactions), clinical reports, data presentation and coding.
It classifies diseases, diagnoses, signs, symptoms, therapeutic indications, investigations and procedures, and medical/social/family history. It doesn’t cover drugs (except as an associated adverse event), equipment or device terminology (except as a device failure), numerical values, study designs or demographics.
Why do we need it?
MedDRA ensures global standardisation of terminology by regulatory agencies – the FDA, EMA and PMDA (Japan) –, pharmaceutical companies and clinicians. And translators.
When was it set up and by whom?
In the late 1980s, a single medical terminology became a pressing need with the introduction of electronic databases. By the 1990s, standardised terminology to support the European Community drug regulatory system became even more urgent.
In 1994, the ICH M1 Expert Working Group created MedDRA for terminology in regulatory affairs. The dictionary was based on the medical terminology implemented by the UK Medicines Control Agency (which later became the MHRA).
MedDRA Version 2.0, released in 1997, was the first true MedDRA terminology.
Who maintains it?
MedDRA is maintained by the MedDRA Maintenance and Support Services Organization (MSSO). This living database is updated twice a year, to ensure it reflects current usage. Non-current terms are also listed for legacy purposes, which can be relevant for translators.
How is it structured?
MedDRA is structured by System Organ Class (SOC). An international order is followed, since alphabetical order would be inconsistent when localised in different languages:
- Infections and infestations
- Neoplasms benign, malignant and unspecified (incl cysts and polyps)
- Blood and lymphatic system disorders
- Immune system disorders
- Endocrine disorders
- Metabolism and nutrition disorders
- Psychiatric disorders
- Nervous system disorders
- Eye disorders
- Ear and labyrinth disorders
- Cardiac disorders
- Vascular disorders
- Respiratory, thoracic and mediastinal disorders
- Gastrointestinal disorders
- Hepatobiliary disorders
- Skin and subcutaneous tissue disorders
- Musculoskeletal and connective tissue disorders
- Renal and urinary disorders
- Pregnancy, puerperium and perinatal conditions
- Reproductive system and breast disorders
- Congenital, familial and genetic disorders
- General disorders and administration site conditions
- Investigations
- Injury, poisoning and procedural complications
- Surgical and medical procedures
- Social circumstances
- Product issues
(Note the new SOC Product Issues at the bottom of the list.)
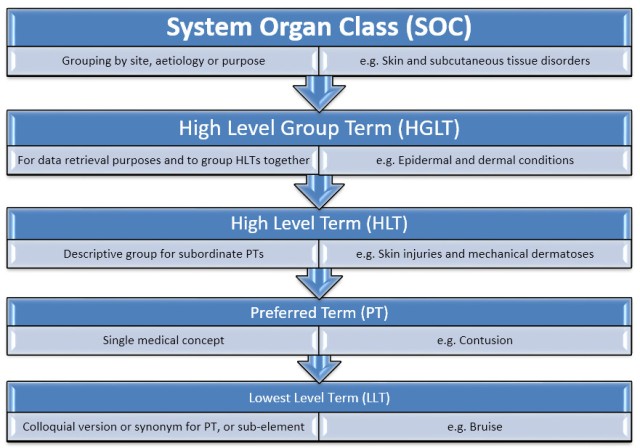
The SOC sits at the top of the hierarchical structure and terms are then subgrouped by HLGT, HLT, PT and LLT (see the chart below for the meanings of these abbreviations). Preferred terms (PTs) are designated as such because they unambiguous, specific and self-descriptive.
Terms may belong to more than one SOC, e.g., haematemesis is coded under Gastrointestinal Disorders and Vascular Disorders.
Example of the bilingual (EN-ES) hierarchical path for the LLT Allergic asthma:
How and when should translators use it?
The master version is published in English and translated into Chinese, Czech, Dutch, French, German, Hungarian, Italian, Japanese, Portuguese and Spanish.
Since 2003, MedDRA terminology has been mandatory in many fields.
Lowest level terms should be used in EU documents for clinical trials (e.g., in Suspected Unexpected Serious Adverse Reactions) and post-marketing documents (in Individual Case Safety Reports and Periodic Safety Update Reports).
Preferred terms should be used in Section 4.8 on Undesirable Effects of the Summary of Product Characteristics.
To make sure I use MedDRA terminology consistently, I add terms to my Multiterm termbase in SDL Trados Studio 2015, and classify them as PTs or LLTs in a context field entry:
How to access MedDRA
An individual subscription to MedDRA costs $190 per year.
Members of Tremédica are entitled to group access to the MedDRA online and desktop browsers.
The online version has improved considerably in recent years and now offers a multilingual display, but it has a session timeout issue and if you’re working on a translation, you’ll have to log on repeatedly. Also, the online version doesn’t remember your settings, so you have to customise language preferences every time.
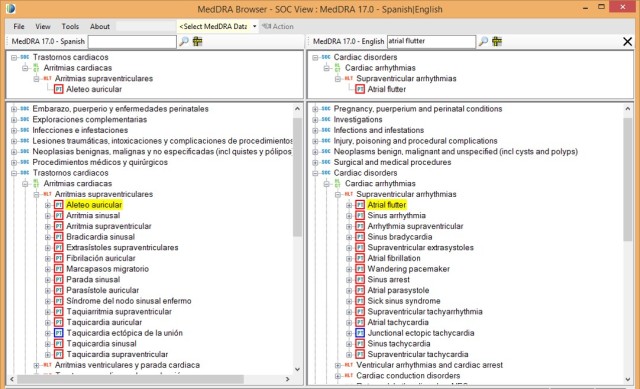
The old desktop browser, 3.1, provided an excellent side by side bilingual display:
(Click the image to enlarge)
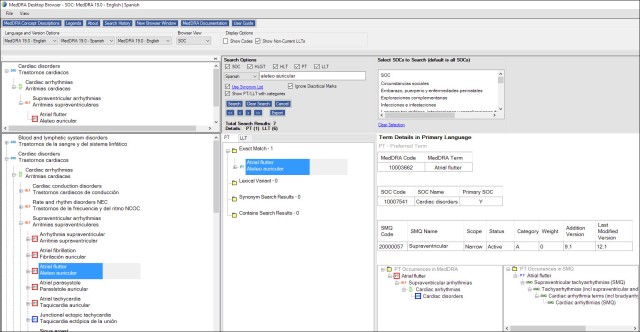
The newer desktop version, 4.0, released in October 2015, has a vertical bilingual/trilingual layout and more features that make it very similar to the online version, without the login and customisation issues:
(Click the image to enlarge)
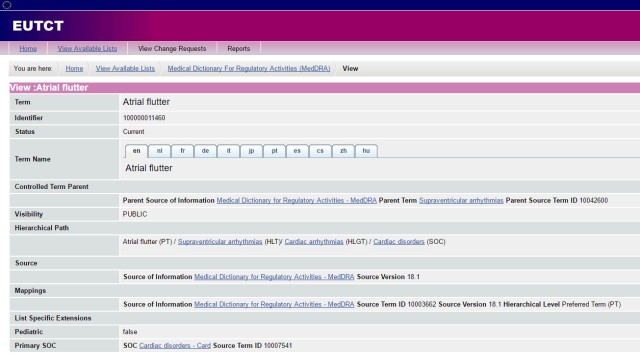
Since 2009, the EUTCT (European Union Telematics Controlled Terms) has provided free guest access to its huge repository, which includes the current multilingual MedDRA databases facilitated through the EUTCT interface.
This interface has a more limited display, because the hierarchical path is in English only and there’s no expandable tree structure. Navigation is less intuitive than the native MedDRA application. But it’s a valid, free alternative.
(Click the image to enlarge)
This whirlwind tour doesn’t do justice to the powerful terminology resource that MedDRA is, but I hope it provides a useful introduction to an essential tool in the medical translator’s repertoire.
References:
MedDRA Introductory Guide Version 19.0
Copyright notices:
MedDRA® the Medical Dictionary for Regulatory Activities terminology is the international medical terminology developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).
MedDRA® trademark is owned by IFPMA on behalf of ICH.





Excellent post, Emma – really well structured and a valuable overview. I especially like the way you’ve included translator-specific info, like how you add the terms to MultiTerm. The link to the free EUTCT service is very useful, too.
Thanks, Jayne. I’m glad you like the MultiTerm part. I added it to the blog post at the last minute, because I was in two minds about it. I always add MedDRA terms to my termbase, but I’m aware terms can become reclassified as non-current or even be changed, so although it’s useful for consistency reasons, it’s best to check back in the current MedDRA version from time to time.
That’s an important point – good that you mentioned it. 🙂
Hi Emma, I really enjoy your posts although my source language is Swedish. Do you know how MedDRA and SNOMED CT relate to each other? Snomed has been translated to Swedish, but I can’t find any Swedish translation of MedDRA.
Interesting question, Johanna. As far as I know, there isn’t a direct way to map one terminology to the other. From a language perspective, as you point out, they’re available in quite different languages, with English and Spanish being the only common denominators. I see that SNOMED CT is only published in English, Spanish, Danish and Swedish. I do use it for reference but not for adverse reactions, which is where MedDRA is strongest (and mandatory). That’s clearly a problem for medical translators like you who have other languages. Kasia Slobodzian-Taylor mentioned this on Twitter yesterday in fact. She’d like to see Polish included in MedDRA.
Thank you! I appreciate it, I would have probably missed this change if it hadn’t been for your blog post. I suppose Appendix II will have to change accordingly.
Thanks for your comment, Ioana. Yes, I expect the EMA will add the new SOC in its next product information update.
Excellent, informative post, as always, Emma. Thanks!
Detailed and well-documented article as usual. Thanks Emma for sharing your tips and know-how.
Thanks for sharing this Emma. Your post is well written and extremely useful for medical translators! Thank you!
Pingback: Weekly translation favorites (Mar 18-24)
Hi Emma,
Eye-opening post as always! You mentioned that members of Tremédica are entitled to group access to the MedDRA online and desktop browsers. Do you know if this is just for the EN-ES language pair, or for all available languages?
Hi Tom, Tremédica members are given access to all languages, including Japanese and Chinese.
The MedDRA subscription rates (through MedDRA MSSO) are $190 for all European languages plus an additional $50 for Chinese or $850 for Japanese.
Thanks, Emma!